The beginning of any complex or challenging endeavor is always the hardest part. Not all of us wake up and jump out of bed ready for the day. Some of us, like me, need a little extra energy to transition out of sleep and into the day. Once I’ve had a cup of coffee, my energy level jumps and I’m good for the rest of the day. Chemical reactions work in much the same way. They need their coffee, too. We call this activation energy.
Understanding how this works can be a useful perspective as part of our latticework of mental models.
Whether you use chemistry in your everyday work or have tried your best not to think about it since school, the ideas behind activation energy are simple and useful outside of chemistry. Understanding the principle can, for example, help you get kids to eat their vegetables, motivate yourself and others, and overcome inertia.
How Activation Energy Works in Chemistry
Chemical reactions need a certain amount of energy to begin working. Activation energy is the minimum energy required to cause a reaction to occur.
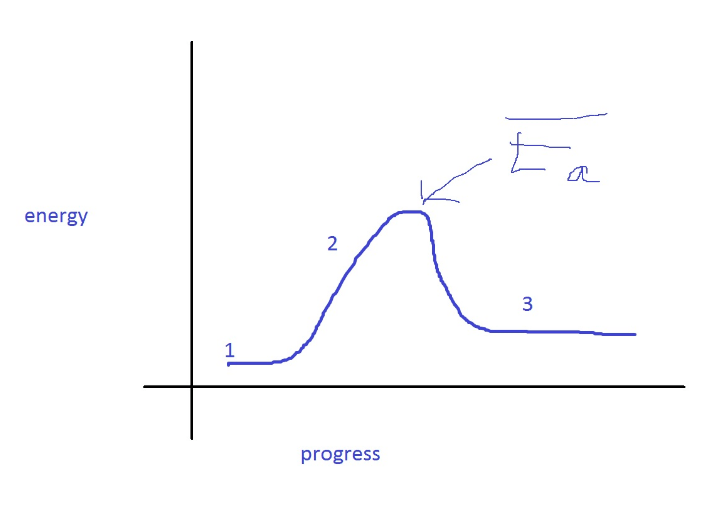
To understand activation energy, we must first think about how a chemical reaction occurs.
Anyone who has ever lit a fire will have an intuitive understanding of the process, even if they have not connected it to chemistry.
Most of us have a general feel for the heat necessary to start flames. We know that putting a single match to a large log will not be sufficient and a flame thrower would be excessive. We also know that damp or dense materials will require more heat than dry ones. The imprecise amount of energy we know we need to start a fire is representative of the activation energy.
For a reaction to occur, existing bonds must break and new ones form. A reaction will only proceed if the products are more stable than the reactants. In a fire, we convert carbon in the form of wood into CO2 and is a more stable form of carbon than wood, so the reaction proceeds and in the process produces heat. In this example, the activation energy is the initial heat required to get the fire started. Our effort and spent matches are representative of this.
We can think of activation energy as the barrier between the minima (smallest necessary values) of the reactants and products in a chemical reaction.
The Arrhenius Equation
Svante Arrhenius, a Swedish scientist, established the existence of activation energy in 1889.
Arrhenius developed his eponymous equation to describe the correlation between temperature and reaction rate.
The Arrhenius equation is crucial for calculating the rates of chemical reactions and, importantly, the quantity of energy necessary to start them.
In the Arrhenius equation, K is the reaction rate coefficient (the rate of reaction). A is the frequency factor (how often molecules collide), R is the universal gas constant (units of energy per temperature increment per mole), T represents the absolute temperature (usually measured in kelvins), and E is the activation energy.
It is not necessary to know the value of A to calculate Ea as this can be figured out from the variation in reaction rate coefficients in relation to temperature. Like many equations, it can be rearranged to calculate different values. The Arrhenius equation is used in many branches of chemistry.
Why Activation Energy Matters
Understanding the energy necessary for a reaction to occur gives us control over our surroundings.
Returning to the example of fire, our intuitive knowledge of activation energy keeps us safe. Many chemical reactions have high activation energy requirements, so they do not proceed without an additional input. We all know that a book on a desk is flammable, but will not combust without heat application. At room temperature, we need not see the book as a fire hazard. If we light a candle on the desk, we know to move the book away.
If chemical reactions did not have reliable activation energy requirements, we would live in a dangerous world.
Catalysts
Chemical reactions which require substantial amounts of energy can be difficult to control.
Increasing temperature is not always a viable source of energy due to costs, safety issues, or simple impracticality. Chemical reactions that occur within our bodies, for example, cannot use high temperatures as a source of activation energy. Consequently, it is sometimes necessary to reduce the activation energy required.
Speeding up a reaction by lowering the activation energy required is called catalysis. This is done with an additional substance known as a catalyst, which is generally not consumed in the reaction. In principle, you only need a tiny amount of catalyst to cause catalysis.
Catalysts work by providing an alternative pathway with lower activation energy requirements. Consequently, more of the particles have sufficient energy to react. Catalysts are used in industrial scale reactions to lower costs.
Returning to the fire example, we know that attempting to light a large log with a match is rarely effective. Adding some paper will provide an alternative pathway and serve as a catalyst — firestarters do the same.
Within our bodies, enzymes serve as catalysts in vital reactions (such as building DNA.)
“Energy can have two dimensions. One is motivated, going somewhere, a goal somewhere, this moment is only a means and the goal is going to be the dimension of activity, goal oriented-then everything is a means, somehow it has to be done and you have to reach the goal, then you will relax. But for this type of energy, the goal never comes because this type of energy goes on changing every present moment into a means for something else, into the future. The goal always remains on the horizon. You go on running, but the distance remains the same.
— Osho, Tantra
No, there is another dimension of energy: that dimension is unmotivated celebration. The goal is here, now; the goal is not somewhere else. In fact, you are the goal. In fact, there is no other fulfillment than that of this moment–consider the lilies. When you are the goal and when the goal is not in the future, when there is nothing to be achieved, rather you are just celebrating it, then you have already achieved it, it is there. This is relaxation, unmotivated energy.”
Applying the Concept of Activation Energy to Our Daily Lives
Although activation energy is a scientific concept, we can use it as a practical mental model.
Returning to the morning coffee example, many of the things we do each day depend upon an initial push.
Take the example of a class of students set an essay for their coursework. Each student requires a different sort of activation energy for them to get started. For one student, it might be hearing their friend say she has already finished hers. For another, it might be blocking social media and turning off their phone. A different student might need a few cans of Red Bull and an impending deadline. Or, for another, reading an interesting article on the topic which provides a spark of inspiration. The act of writing an essay necessitates a certain sort of energy.
Getting kids to eat their vegetables can be a difficult process. In this case, incentives can act as a catalyst. “You can’t have your dessert until you eat your vegetables” is not only a psychological play on incentives; it also often requires less energy than constantly fighting with the kids to eat their vegetables. Once kids eat a carrot, they generally eat another one and another one. While they still want dessert, you won’t have to remind them each time, so you’ll save a lot of energy.
The concept of activation energy can also apply to making drastic life changes. Anyone who has ever done something dramatic and difficult (such as quitting an addiction, leaving an abusive relationship, quitting a long-term job, or making crucial lifestyle changes) knows that it is necessary to reach a breaking point first. The bigger and more challenging an action is, the more activation energy we require to do it.
Our coffee drinker might crave little activation energy (a cup or two) to begin their day if they are well rested. Meanwhile, it will take a whole lot more coffee for them to get going if they slept badly and have a dull day to get through.
Conclusion
To understand and use the concept of activation energy in our lives does not require a degree in chemistry. While the concept as used by scientists is complex, we can use the basic idea.
It is no coincidence that many of most useful mental models in our latticework originate from science. There is something quite poetic about the way in which human behavior mirrors what occurs at a microscopic level.
For other examples, look to Occam’s Razor, falsification, feedback loops, and equilibrium.
